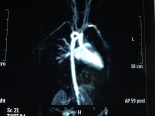
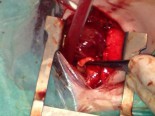
Çocuk Kalp Hastalıkları ve Cerrahisi (Pediatric Heart Diseases and Cardiac Surgery)
Çocuk Kalp Cerrahisi Platformu (Platform for Pediatric Heart Surgery)
Pediatrik Kalp Hastalıkları ve Cerrahisi
Pediatric Heart Diseases and Cardiac Surgery
Tek ventrikül
Triküspid Atrezisi
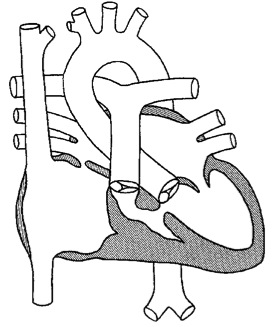
> 4 ünite – cerrahi müdahale kontrendikedir
2-4 ünite – Bidirectional kavopulmoner şant (Glenn şant): Superior vena kava ile sağ pulmoner arter arası bağlantı kurularak sağlanır.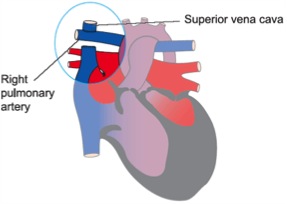
< 2 ünite – Total Kavopulmoner şant (Fontan operasyonu): Glenn şant yapılmış hastada inferior vena kavanın sağ pulmoner arterle birleştirilmesi ile veya ilk palyasyon yapılanlarda hem SVC hem IVC’nin sağ pulmoner arterle birleştirilmesi ile gerçekleştirilir.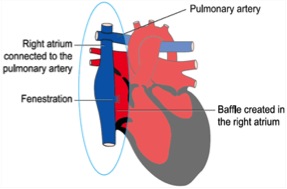
Doç.Dr.Buğra Harmandar
Single Ventricle
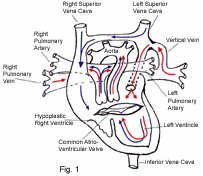
Pathophysiology
The term single ventricle refers to any congenital cardiac anomaly in which one ventricle is hypoplastic or absent, such as hyposplastic left heart syndrome. Hypoplasia of either semilunar valve or either ventricular outflow tract is generally amenable to a biventricular repair. In contrast, significant hypoplasia of either atrioventricular valve, or of the apical portion of either ventricle generally necessitates a single ventricle approach. Early diagnosis and management in the newborn period or early infancy is essential.
Surgical Technique
Initial efforts are directed at complete relief of systemic outflow tract obstruction and limitation of pulmonary blood flow to prevent development of elevated pulmonary vascular resistance. The ultimate goal is Fontan circulation in which systemic venous return is directed to the pulmonary arteries without an intervening pumping chamber. Low pulmonary vascular resistance is essential in order that blood will flow to the pulmonary arteries passively at an acceptable venous pressure. Fontan circulation is usually accomplished in two stages. Initially, at four to six months of age, a cavopulmonary shunt is constructed which directs superior vena caval blood flow to the confluent pulmonary arteries. Fontan completion is accomplished by redirecting inferior vena caval blood flow to the pulmonary arteries with an intracardiac baffle or extracardiac conduit (See diagram). Fontan completion is usually undertaken at approximately two years of age.
Cardiopulmonary bypass is required for the Fontan completion procedure, and is usually utilized for the cavopulmonary shunt procedure as well. Use of aortic cross-clamping is variable and depends upon the individual anatomy and surgeon preference.
Postoperative Considerations
The postoperative course following the cavopulmonary shunt procedure is usually uncomplicated. The postoperative course following completion Fontan can be more complex. Invasive monitors utilized following repair include arterial, central venous and left atrial catheters. Vasoactive infusions required for hemodynamic management might include dopamine or dobutamine, epinephrine, milrinone and nitroprusside.
Special postoperative consideration is given to careful monitoring of transpulmonary gradient (central venous pressure - left atrial pressure). In addition to transpulmonary gradient, special attention is given to careful monitoring of central venous pressure as well as evidence of systemic venous congestion. Central venous pressure above fifteen to eighteen mm Hg or transpulmonary gradient above ten mm Hg indicates difficulty with passive flow of blood across the pulmonary capillary bed. Physical exam findings in these patients might include hepatomegaly, abdominal ascites, and edema of the head and neck (cavopulmonary shunt) or anasarca. Restriction of the free flow of blood from systemic venous return, through the pulmonary vascular capillary bed to the left atrium results in reduction of cardiac output following the Fontan or cyanosis following the cavopulmonary shunt. Hemodynamic changes must be monitored especially closely following Fontan completion. In contrast to patients with biventricular physiology, patients with univentricular physiology do not have as much hemodynamic reserve, especially during the first few days following surgery. Small increases in pulmonary vascular resistance can result in decreased cardiac output.
Sinus node dysfunction is common following Fontan completion. Extensive atrial suture lines and alteration of normal intra-atrial pressures can lead to a loss of sinus rhythm. Loss of normal atrioventricular synchrony and severe tachycardia are poorly tolerated. Implantation of a permanent pacemaker is occasionally required.
Arterial oxygen saturation following Fontan completion may be slightly low (eighty-eight to ninety-three percent) since the coronary sinus is not included in the Fontan anastomosis. This coronary sinus blood return is allowed to mix with pulmonary venous return, thus leaving a small right-to-left shunt. Arterial oxygen saturation following cavopulmonary shunting should be seventy-five to eighty percent.
Postoperative pleural effusion is common, especially following Fontan completion. Chest tubes are routinely placed in both pleural spaces during Fontan surgery. Persistence of pleural drainage for days and sometime weeks can occur. Chemical pleural sclerosis is sometimes required to control protracted pleural drainage. Extended pleural drainage following cavopulmonary shunting occurs less commonly, but is occasionally encountered.
Anticoagulation is usually initiated following Fontan completion surgery. Slow venous blood flow through long segments of prosthetic material make these patients especially susceptible to thrombus formation in the early postoperative period. Coumadin is usually started once intracardiac lines have been removed. Anticoagulation following cavopulmonary shunting is usually less rigorous and might include aspirin or dipyridamole depending upon cardiologist and surgeon preference. Usual pre-discharge medications include digoxin, lasix and captopril.
Length of hospital stay required following the bidirectional cavopulmonary shunt procedure averages five to seven days. Length of hospital stay required following the Fontan completion operation is less predictable. Ten days to three weeks is average.
Yorumlar - Yorum Yaz